Customized PEGylation Services (Collaboration with Biosynth GmbH)
From Early Development to Commercial Production
PEGylation has been an established technology since 1990. Nevertheless, the development of a PEGylated drug is challenging and requires extensive experience in both chemistry and biochemistry. Only a few companies worldwide have sufficient know-how and resources to successfully perform such development on their own.
NOF and Biosynth GmbH offer customized PEGylation services from early development through clinical, up to commercial stage on a fee-for-service basis. By utilizing this service, customers can benefit from:
・ More than 30 years of experience in the development of PEGylated drugs
・ Combined expertise in chemistry and biologics
・ Increased speed of their PEGylation development
・ Minimizing the risk of failure in PEGylation
・ Saving own resources for API development
・ Continued consistent supply of highly pure activated PEG products from research through clinical development and commercial stage
Customers may select between single service modules according to their individual needs or a complete development package. A complete development project comprises five steps and takes 12-18 months from early screening to clinical GMP-production.
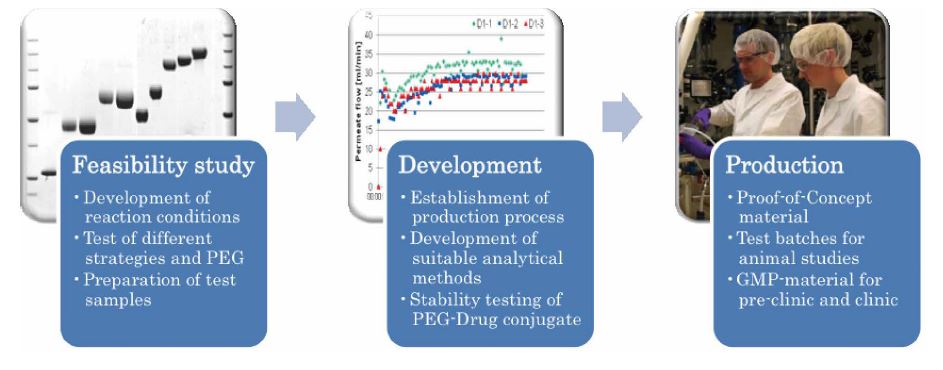
Figure: Service modules for the development of PEGylated drugs
