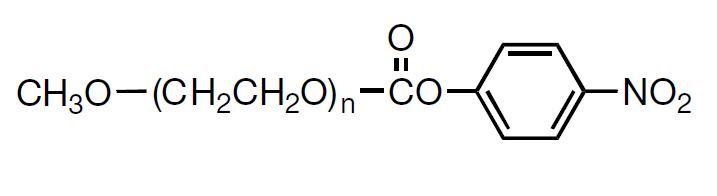NOF has a long reputation for producing high-quality Activated PEGs -the SUNBRIGHT® Series and high-quality monodisperse PEGs -the PUREBRIGHT® Series that possess the most suitable properties for preparation of physiologically active drugs and biologics. Our SUNBRIGHT® Series and PUREBRIGHT® Series is characterized as extremely high-purity PEGs with a variety of functional groups. The products of this series are internationally recognized as optimal PEGylated drugs. As illustrated in this figure, application of Activated PEGs with various functional groups enables introduction of PEG chains into drugs, enzymes, phospholipids and other biologics. Covalent conjugation of hydrophobic macromolecules with Activated PEGs leads to the formation of macromolecular micelles (Polymer Micelles), which allow homogeneous dispersion of hydrophobic drugs in aqueous media.
Currently, monodisperse PEGs the PUREBRIGHT® Series are used in Antibody Drug Conjugates (ADC) drugs as a linker to increase their hydrophilicity.
PEGylation plays an important role in the stabilization of drugs, increased circulation time, reduction of their antigenicity and decrease in drug dosage, besides augmenting the targeting ability via binding biologics onto their surfaces.

High-purity mPEG-OH: Starting Material for Activated PEGs
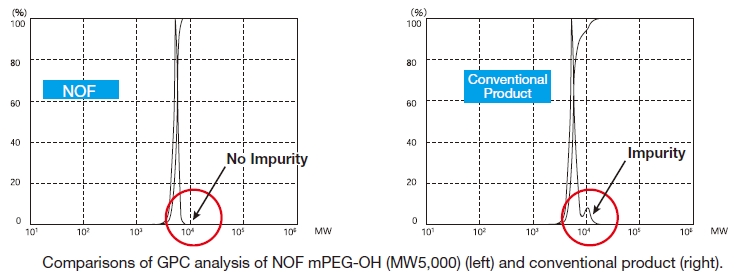
NOF manufactures high-purity methoxy-PEG-OH characterized by absence of contaminating impurities and narrow molecular weight distributions.
These highly pure methoxy-PEG-OH have so far been used for various commercial PEGylated drugs, such as PEGInterferon and PEG-GCSF.
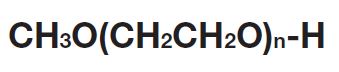
Our methoxy-PEG-OH has the advantage of extremely low diol contents relative to those of competitors’ products. As shown in the following charts, our methoxy-PEG-5000 contains remarkably low levels of diol as impurities, besides providing a narrower distribution of molecular weights in comparison with our competitors’ products. Our methoxy-PEG-OH is also employed as a starting material of our Activated PEG products, which contributes the high purity and high activity of the Activated PEG. We manufacture highly pure methoxy- PEGs with molecular weights from 2 kDa to 80 kDa.
About PEGylation
PEGylation technology is applied for biologics modifications including cytokines, therapeutic antibody fragments, interleukins, hormones, oligonucleotides and other proteins and peptides.
PEGylation can be effective for :
•Improving Bioavailability •Prolonging Blood Circulation
•Maximizing Pharmacokinetics •Low-profile Immunogenicity
NOF provides high-performance activated PEGs for PEGylated drugs from early development stage to commercial use with over 30 years of experience of manufacturing high-quality methoxy polyethylene glycol. As the starting material for activated PEGs for pharmaceuticals, our activated PEGs have a narrow polydispersity and low diol content with wide range of molecular weights, from 2kDa to 80kDa.
PEGylation Service
Please feel free to contact us if you need PEGylated drugs using NOF Activated PEGs, SUNBRIGHT® Series. Contact form
Capabilities
cGMP Manufacturing Facilities
NOF Activated PEGs, SUNBRIGHT® Series, are produced in facilities using state-of-the-art technology, operated under cGMP. The cGMP facilities are capable of producing small to large batch sizes, using proprietary know-how with scale-up procedures, depending on customers’ needs.
These cGMP facilities have been audited by pharmaceutical companies from around the world. NOF receives a consistent high reputation from our customers.
Research & Development
Our R&D facility was opened in December 2005. This allows us to continue our development of novel activated PEGs and new technologies for PEGylation.
Analytical Technologies
NOF has more than 30 years of history for manufacturing high-quality mPEGs and Activated PEGs. These experiences endow NOF with a thorough knowledge of PEGs and considerable achievement in analysis of Activated PEGs. We also invented innovative analytical methods for assaying purity of activated PEGs and for determining impurity levels.
Validation Capability
NOF has much experience to establish GMP product with entire Validation steps such as Risk assessment, PPQ production, Analytical validation. Production and supply based on these experiences can be useful at the time of customers’ regulatory submission.
Application Chart for PEG Derivatives
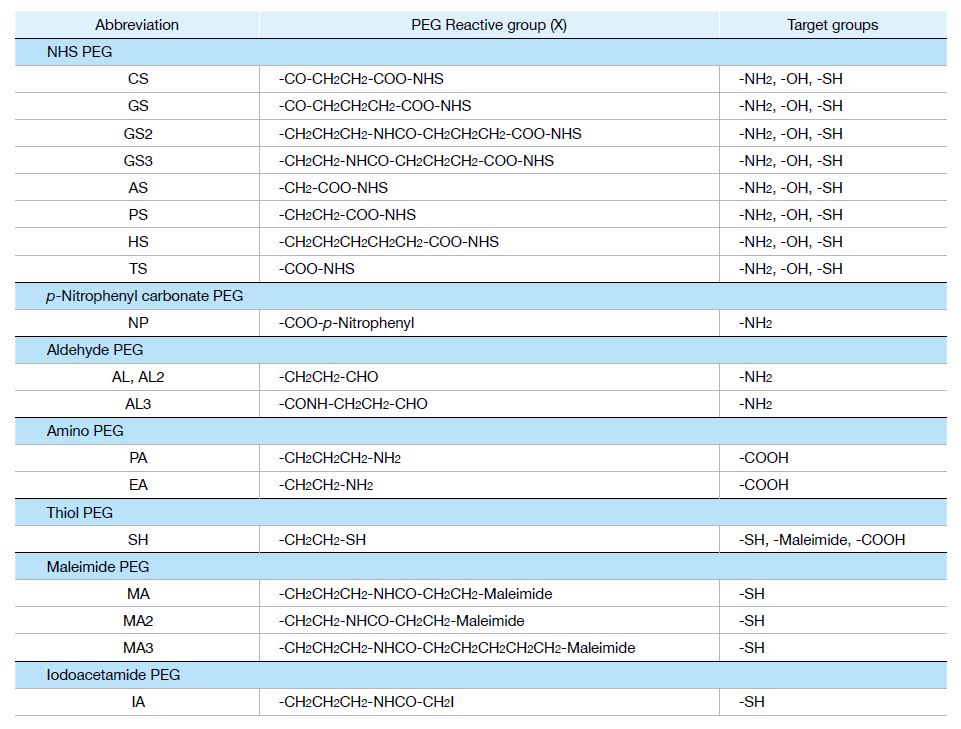
We supply different series of PEG derivatives with various versatile functional groups.
Custom Manufacturing
NOF has more than 30 years of experience in supplying high-quality methoxy polyethylene glycol (mPEG) to pharmaceutical markets. The highest quality mPEG is our starting material of PEG derivatives, patented through our proprietary technology.
Based on our extensive range of technological know-how and capabilities, NOF offers custom manufacturing from early stage development to commercial scale.