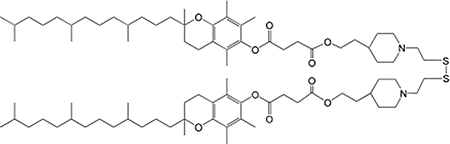
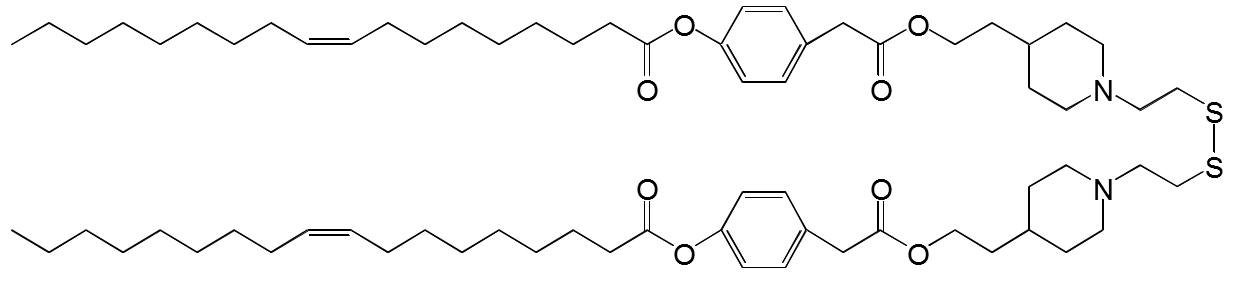
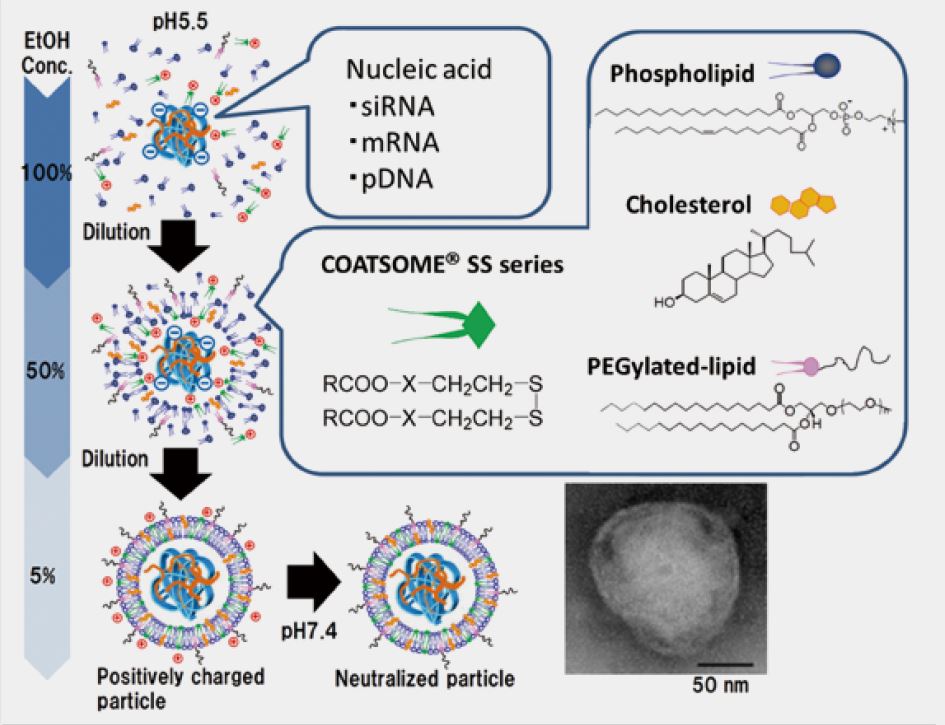
SS-cleavable and pH-responsive lipid-like material for gene, nucleic acids, and small molecule drug delivery system
We have newly developed COATSOME® SS-series for effective intracellular delivery and lower toxicity.
COATSOME® SS-series contains dual sensing motifs that can respond to the intracellular environment; tertiary amines respond to an acidic compartment (endosome/lysosome) for membrane destabilization, and disulfide bond that can cleave in the reductive environment (cytoplasm). These two units synergize for efficient intracellular delivery of encapsulated drugs with enhanced biosafety.

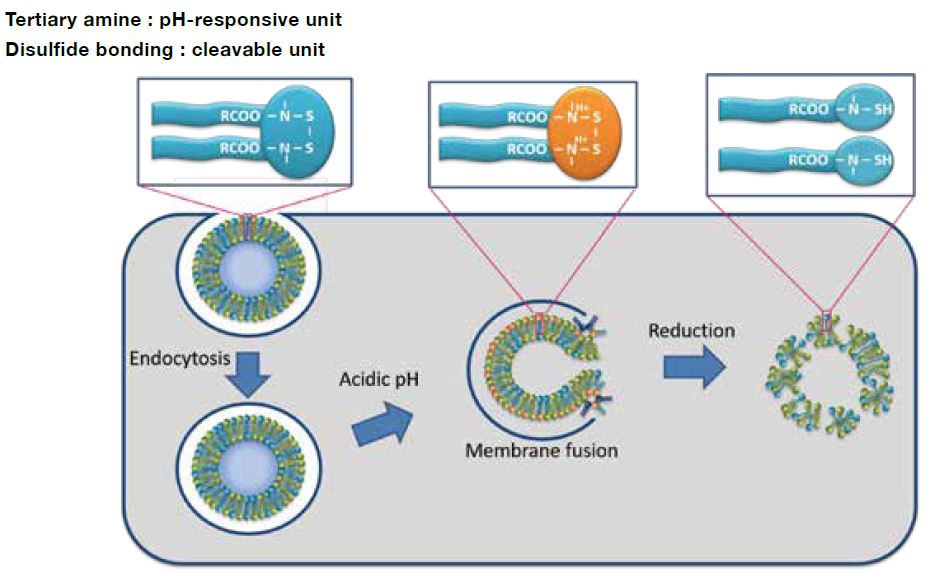
Proof of Concept
Particle formation
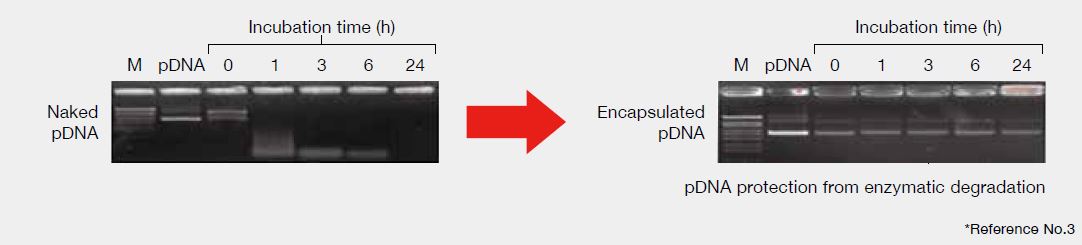
 Serum resistance
Serum resistance
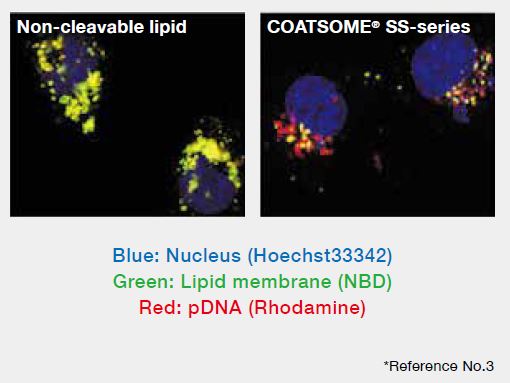
Membrane disruption in the cells
The particle composed COATSOME® SS Series is stable in serum, hence enzymatic degradation of pDNA was prevented by encapsulation.
Also the particle composed of COATSOME® SS Series was effectively destabilized in the cytoplasmic environment.
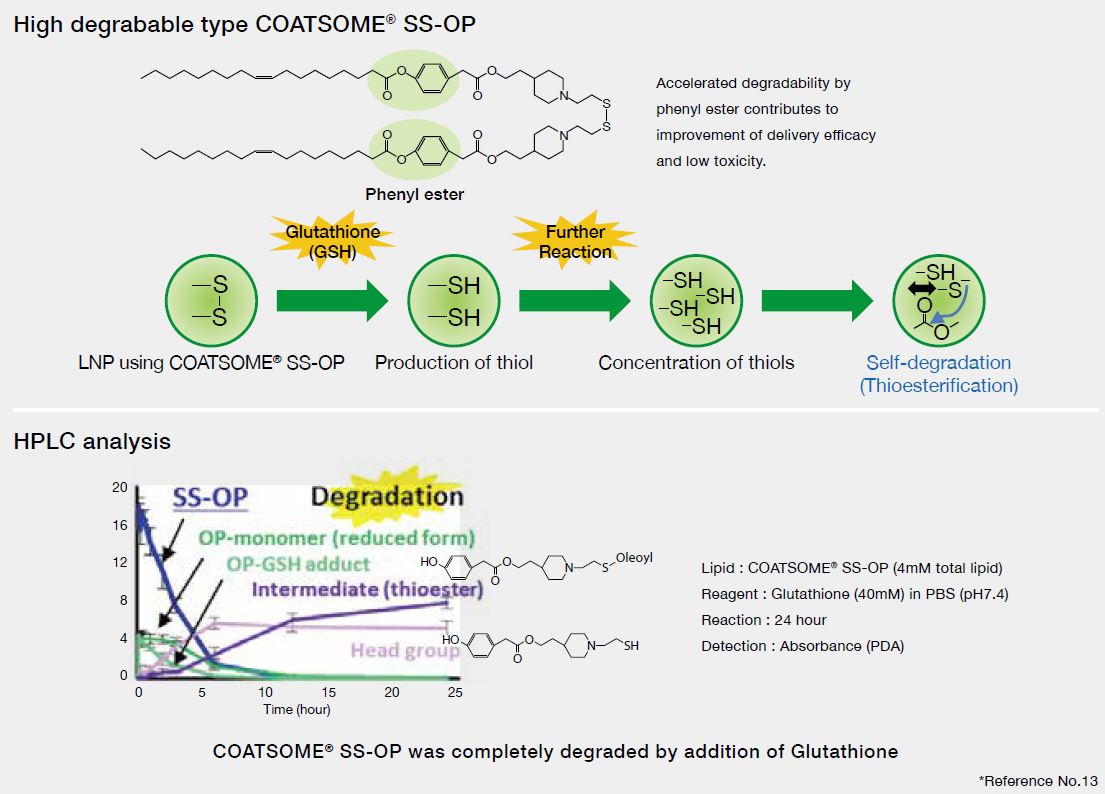
Degradation of COATSOME® SS-OP
In vivo toxicity in comparison to a competitor lipid
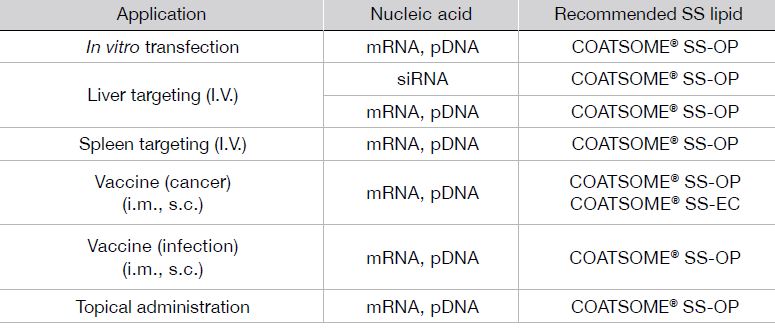
Examples of application
With its high biocompatibility, COATSOME® SS Series enables the intracellular delivery of various compounds with high efficiency. COATSOME® SS Series particles can encapsulate pDNA, siRNA, mRNA and hydrophobic low molecular weight compounds, and has been proven in delivery targeting tumor, spleen, lymph node and brain. In the following we will introduce data on hepatic siRNA delivery, hepatic mRNA delivery data and lymphatic mRNA delivery data for RNA vaccine application as examples.
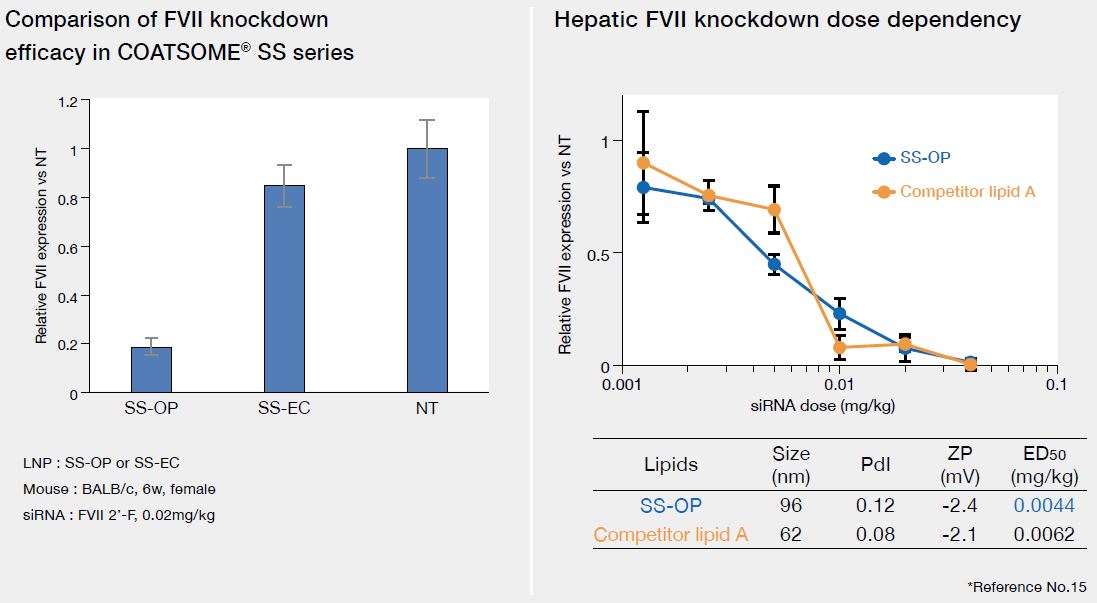
Hepatic delivery of siRNA
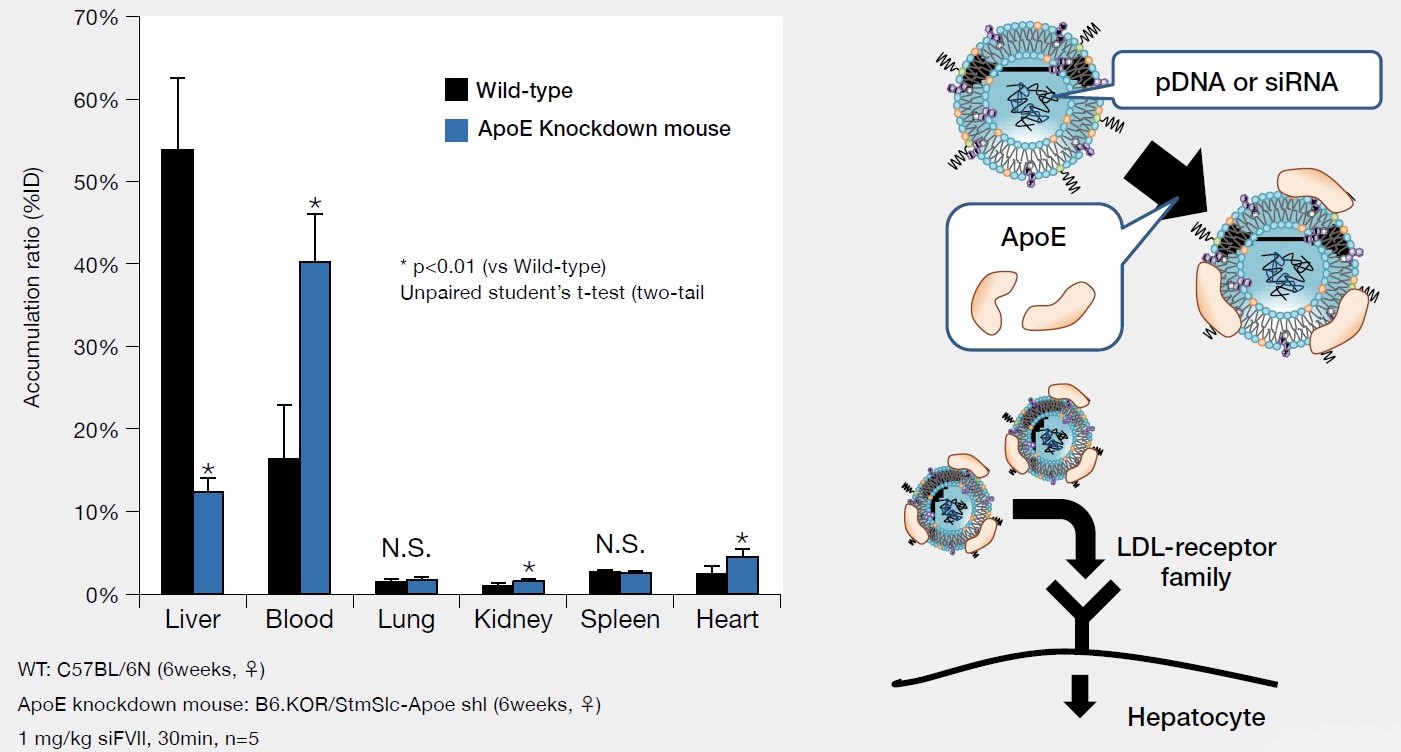
Distribution of [3H] labeled LNP SS-EC
Hepatic delivery of mRNA

Gene editing (CRISPR/Cas9)

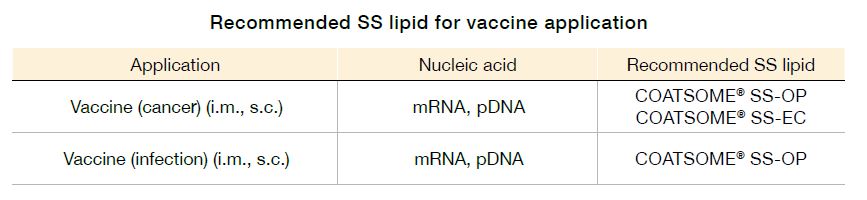
RNA vaccine
CTL activity
 Humoral immunity
Humoral immunity
Therapeutic anti-tumor effect
In vitro transfection of mRNA

Splenic delivery formulation
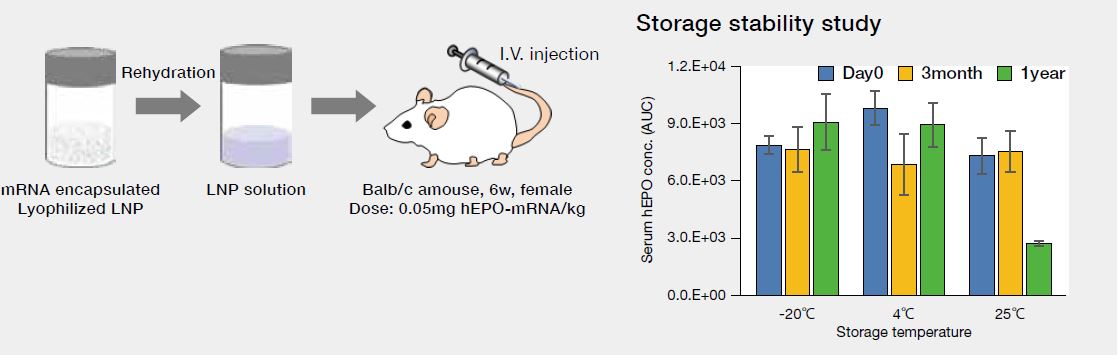
Lyophilized LNP
Feature
• Encapsulation: siRNA, mRNA, pDNA
• Efficient drug delivery into the cytoplasm of cells
• Biodegradability and low toxicity
• Controlled targeting of organs: liver, spleen, or lymph nodes
• Long-term strage of lyophilized LNP at 25℃
• Vaccine application (Humoral and Cellular immunity)
Formulations
Reference List
( 1 ) Hepatic pDNA delivery : M. Ukawa et al., Adv. Healthcare Mater., 3, 1222-1229 (2014)
( 2 ) Tumor pDNA delivery : H. Akita et al., J. Control. Release, 200, 97–105 (2015)
( 3 ) In vitro pDNA delivery : H. Akita et al., Adv. Healthc Mater., 2 (8):1120-5 (2013)
( 4 ) Small molecule drug encapsulated particle : H. Tanaka et al., Colloids Surf. B, 151, 95-101 (2017)
( 5 ) Inflammatory site small molecule delivery (I.V.) : A. Watanabe et al., Int. J. Pharm., 509, (1–2), 118–122 (2016)
( 6 ) Hepatic siRNA delivery : H. Akita et al., ACS Biomater. Sci. Eng., 1 (9), 834–844 (2015)
( 7 ) pDNA delivery to brain (lCV injection) : H. Akita et al., Int. J. Pharm., 490 (1-2), 142-145 (2015)
( 8 ) mRNA delivery to brain (ICV injection) : H. Akita et al. , Mol. Pharm., 15(5), 2060−2067 (2018)
( 9 ) Lung delivery by peptide ligand LNP (I.V.) : S. Santiwarangkool et al., J. Pharm. Sci., 106, 2420-2427 (2017)
(10) DNA vaccine for cancer immunotherapy (S.C.) : H. Akita et al., Nanomedicine, 14(8), 2587-2597 (2018)
(11) Lyophilized LNP encapsulating siRNA : D. Shirane et al., Biol. Pharm. Bull., 41(8): 1291-1294 (2018)
(12) Improvement of gene expression activity by anti-inflammatory drug delivery (I.V. injection) : T. Ohto et al., Biol. Pharm. Bull., 42(2):
299-302 (2019)
(13) Hepatic mRNA delivery by SS-OP LNP : H. Tanaka et al., Adv. Funct. Mater., 191757 (2020)
(14) DNA vaccine for cancer and Protozoan infection : M. Maeta et al., Mol. Pharm., 17(4), 1237-1247 (2020)
(15) Hepatic oligonucleotide delivery : H. Tanaka et al., Pharmaceutics, 13, 544 (2021)
(16) Efficient mRNA transfection in T cell line : H. Tanaka et al., Pharmaceutics, 13(12), 2097 (2021)
(17) Efficient mRNA transfection in brain capillary endothelial cells : Y. Sakurai et al., Pharmaceutics, 14(8), 1560 (2022)
(18) Targeted delivery of LNP to lymphatic endothelial cells : Y. Sakurai et al., J. Control. Release, 349, 379-387 (2022)
(19) siRNA delivery to lymphatic endothelial cells : Y. Sakurai et al., J. Control. Release, 353, 125-133 (2022)
(20) Splenic mRNA delivery by PS loaded LNPs : Y. Sakurai et al., Adv. Healthcare Mater., Dec 19, e2202528 (2022)